ISO9001:2008 certification
Home / Products/Services / Yellow Belt Certification Services / ISO9001:2008 certification
ISO9001:2008 certification
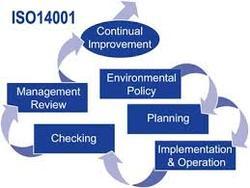
Get Best Quote ISO 13485:2003 Certification Ensure confidence in medical device safety ISO 13485 will help organization do business in this highly regulated sector. Whether organization's are looking to operate internationally or to expand locally to accommodate new business, ISO 13485 will help demonstrate to customers and regulators that they are committed to quality. The regular assessment process will ensure that processes are continually checked for effectiveness and provide the opportunity to avoid unpleasant regulatory surprises. Certification to this standard can improve overall performance, remove uncertainty and widen market opportunities with complying to requirements, such as: Basic Quality System requirements with several enhancements Risk Management Approach to product development and product realization Validation of processes Consideration of statutory and regulatory requirements Tracking and record keeping Assuring positive product traceability and recall Who it is relevant to : ISO 13485 is relevant to all manufacturers of medical devices (including subcontract manufacturers) to satisfy both regulatory and voluntary requirements. Benefits : ISO 13485 or in combination with regulatory certification, will be viewed by organization's customers, distributors and authorities as a genuine commitment to the quality of medical devices during the approval or bidding process. Most medical device manufacturers will eventually want to sell their products worldwide. Certification to this standard will help achieve this objective. Regular assessments performed by Zenith will help organization's to monitor and improve their management system and processes. This improves the reliability of operations and products, ensures compliance with regulatory and customer requirements and increases financial performance.
Send EnquiryRelated Products
Products / Services
- Lead Auditor Training
- Lead Auditor Iso 22001 Fsms/haccp
- Gost R Explosion Proof Certificate
- Internal Auditor Training
- Testing Civil Construction Products
- Ce Marking Services
- Six Sigma Training Institute
- Six Sigma Executive Program
- Six Sigma Green Belt
- Six Sigma Black Belt
- La 9001training
- Six Sigma Training
- Training Program
- Welder Testing Certification
- Internal Auditor Iso 13485
- Internal Auditor Training
- Export To Uganda Pre-export Verification Of Conformity Std
- Internal Auditor Training
- Technical Inspection Tpi
- Security Inspection Machines
- Ship Technical Inspection Tpi
- Containers Technical Inspection Tpi
- Gostr Legal Framework
- Type Of Gost R Certificates
- What Is The Ukrsepro Certificate
- Type Of Ukrsepro Certificates
- Iso 9001 2008 Certification
- Information Technology Iso
- Iso 50001 Energy Management
- Iso 3834 Certification
- View All